Spirovant Sciences Doses First Patient in the SAAVe Phase 1/2 Clinical Trial of Its Investigational, Aerosol-Delivered Genetic Medicine for the Treatment of Cystic Fibrosis

SP-101, an AAV vector-based gene therapy delivered via inhalation, is being investigated in combination with doxorubicin, an augmenter to enhance transgene expression in the lungs Novel approach represents new potential for patients who do not benefit from current treatment options November 14, 2024 Spirovant Sciences, a clinical-stage gene therapy company developing treatments for inherited respiratory […]
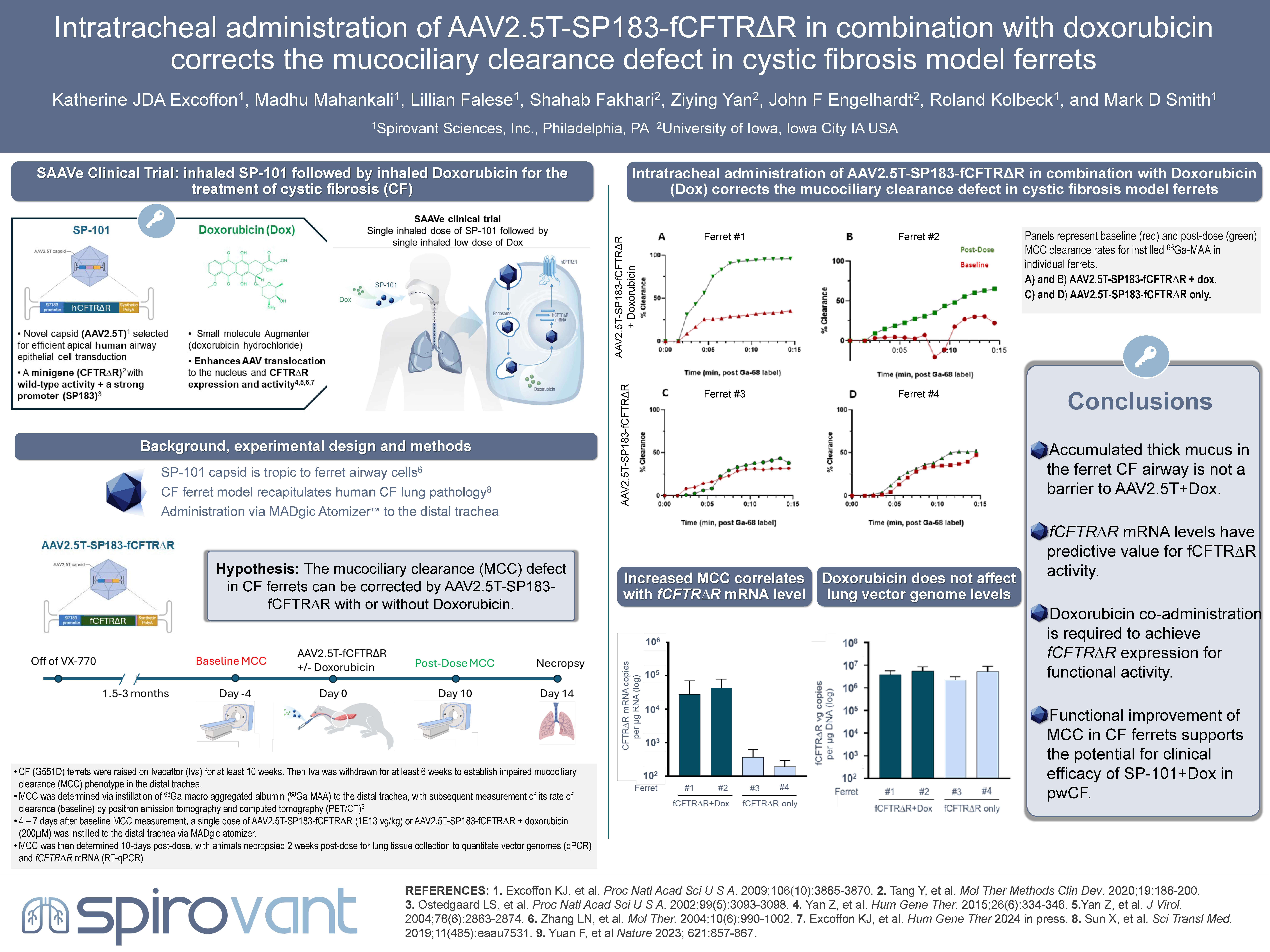
Poster: Intratracheal administration of AAV2.5T-SP183-fCFTRΔR in combination with doxorubicincorrects the mucociliary clearance defect in cystic fibrosis model ferrets
Delivery of SP-101 restores CFTR function in human CF airway epithelial cultures and drives hCFTRΔR transgene expression in the airways of ferrets

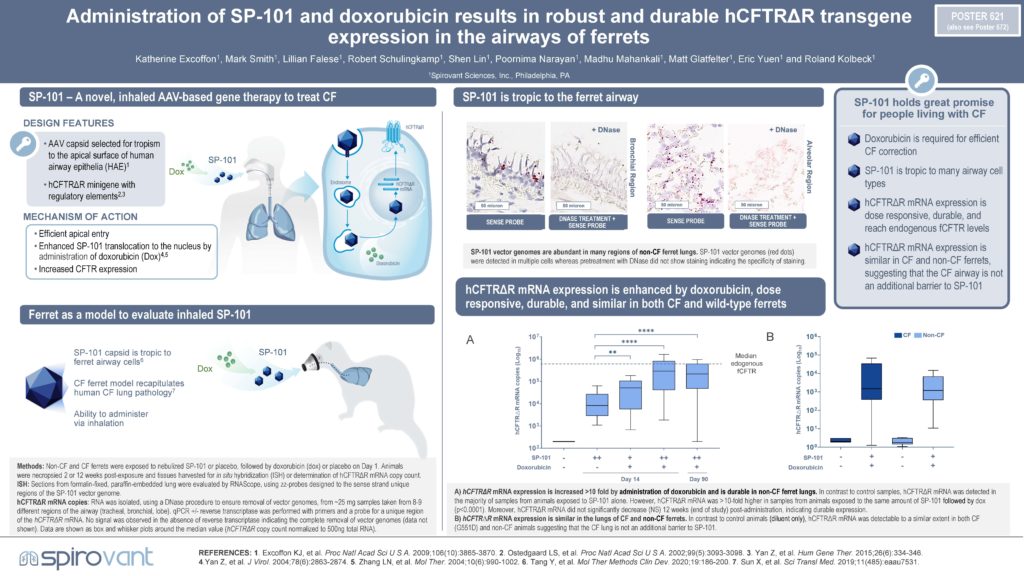
Poster: 621: Administration of SP-101 and doxorubicin results in robust and durable human cystic fibrosis transmembrane conductance regulator minigene transgene expression in the airways of ferrets and corrects human cystic fibrosis airway epithelia in vitro. North American Cystic Fibrosis Conference 2022
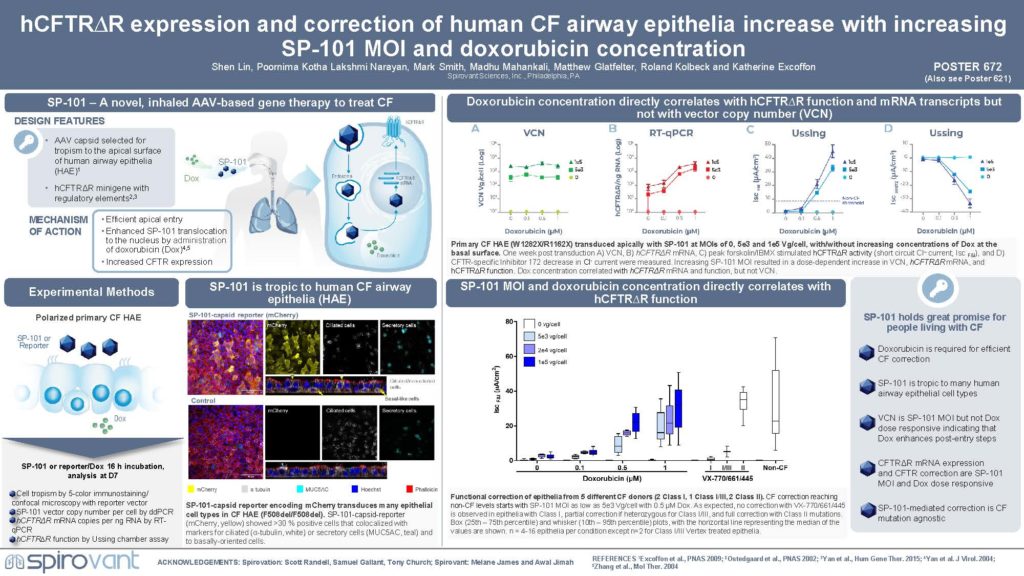
Poster: 672: SP-101 activity increases in a dose dependent manner to both vector and doxorubicin dose. North American Cystic Fibrosis Conference 2022
Spirovant’s SP-101 pre-clinical data demonstrates great promise for individuals with cystic fibrosis (CF) who do not benefit from small molecule modulators

November 3, 2022 New pre-clinical data for SP-101, an investigational novel recombinant adeno-associated virus (AAV) gene therapy selected for its tropism to human airway epithelia for the treatment of people living with cystic fibrosis (CF), suggest co-administration of SP-101 with doxorubicin functionally corrects human CF airway epithelia by restoring chloride conductance to similar levels as […]
Abstract: 672: SP-101 activity increases in a dose dependent manner to both vector and doxorubicin dose. North American Cystic Fibrosis Conference 2022

Abstract: 621: Administration of SP-101 and doxorubicin results in robust and durable human cystic fibrosis transmembrane conductance regulator minigene transgene expression in the airways of ferrets and corrects human cystic fibrosis airway epithelia in vitro. North American Cystic Fibrosis Conference 2022
